Document Pro | Document Version Control Software
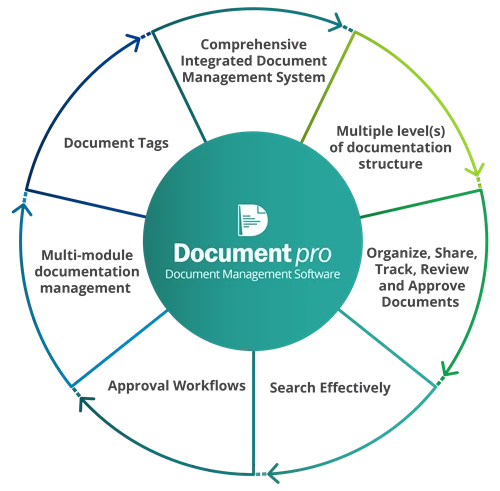
Document Pro is a single repository to manage all your business documents and records. You can monitor employee access, document revisions, change requests, and approvals through an online platform. Documents can be customized into infinite number of levels and folders per structural requirements. The system follows a pre-programmed base structure in which; Level 1 is for Quality Manual, Level 2 for Procedures, and Levels 3 and 4 for Work Instructions and Checklists, respectively. Especially powerful for integrated management systems!
Document Pro software is compliant with the following standards:
- ISO 9001
- IATF 16949
- Aerospace AS9145
- FDA CFR 21 Part 11
- ISO 13485
- ISO 14001
- ISO 45001
- ISO 27001
- FSSC 22000

Omnex Systems has successfully completed the SOC 2 Certification, reinforcing our commitment to maintaining the highest standards of data security and privacy for our clients.
View All certifications